Le groupe Métier de la Recherche Clinique sera présent au forum BIOTechno pour présenter les différents métiers du secteur ainsi que les perspectives d’emploi.
📅 Lundi 5 octobre 8h45
📍 Espace Charenton, 1 Rue Théodore-Hamont, 75012 Paris
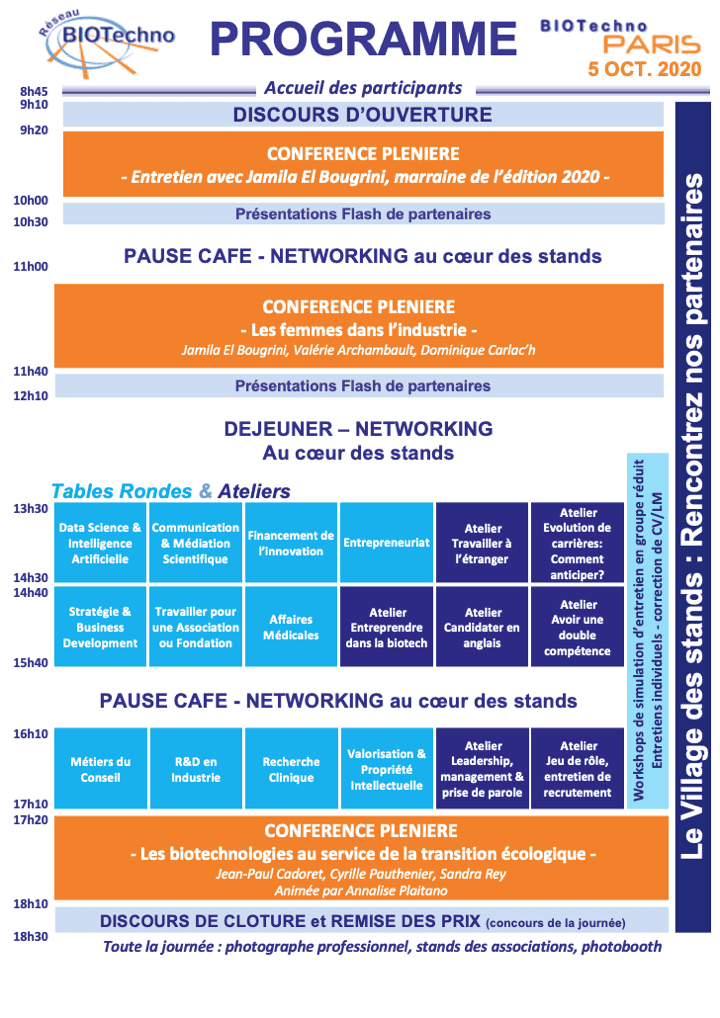
Le groupe Métier de la Recherche Clinique sera présent au forum BIOTechno pour présenter les différents métiers du secteur ainsi que les perspectives d’emploi.
📅 Lundi 5 octobre 8h45
📍 Espace Charenton, 1 Rue Théodore-Hamont, 75012 Paris

In this 1-hour webinar, you will learn why computerized systems must go through a validation process when used in clinical trials, as well as the consequences of failing to validate your systems. We will explain the requirements for validation, who is responsible and when it is necessary. Through the speaker’s experience, you will learn the why, what, and how of validation with regards to regulatory requirements.
Continue reading